What is pH?
pH is the measurement of whether something is acidic or alkaline (base). The number scale used to measure pH is from 1 to 14 with 1 being the most acidic and 14 being the most alkaline (base). 7 is considered neutral, neither acid or base. The normal pH of rain water is not 7, the neutral number, as you might think. It is actually about 5.6, which is acidic. This is because the rain combines with carbon dioxide in our air. This makes a very mild form of carbonic acid naturally.
In the Adirondacks and throughout the northeastern US and SE Canada however, the pH of falling rain can be as low as 4 or 5. This is much more acid than normal. Each number represents 10 times the acidity of the number before it. So if rain water goes from the normal 5.6 to 4.6 it is 10 times more acid than normal.
What makes acid rain?
The burning of coal, oil and gasoline gives off sulfur, nitrogen and carbon that combine with water vapor in the air to make acids, like sulfuric acid, nitric acid and carbonic acid. These mild acids fall to earth as rain or snow. When they land in lakes and streams they can make the water so acid that fish and frog eggs won’t hatch. Soon those lakes have no fish or frogs left in them. Acid rain can also cause building and statues to rot away. It can build up in the soil and slow the growth of some trees and kill others.
Where does acid rain falling on rural and wild forests come from?
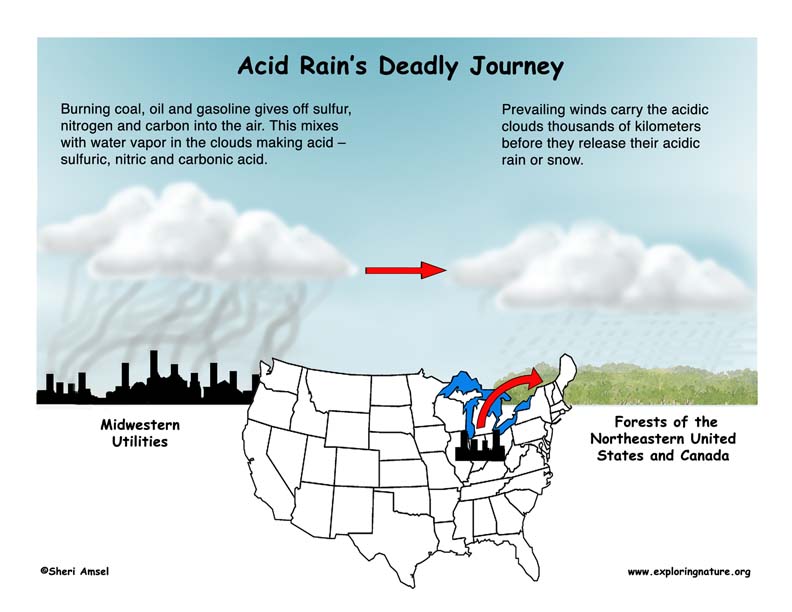
It is carried by the wind. The wind patterns of North America carry pollutants from busy cities, factories and industrial centers in the Midwestern part of the United States and Canada and drops them on the northeast.
Why doesn't the environment protect itself from the acid, like it does with the natural acids in rain?
The soil naturally contains calcium carbonate, a substance that is alkaline, and mixes with the normal acid of rain and as it washes into lakes and stream and cancels the acid effect out. These are called “buffers.” As the rain becomes 10 times more acid than normal the buffers aren’t strong enough to counteract the acid and it flows freely into our waterways.
Is acid rain always man-made?
Not all air pollutants are man made. A volcanic eruption can push millions of tons of acidic debris into the air and have a similar effect on the rain and snow. Though intense following a volcanic eruption, this effect is not long term in this case.
Purpose of Activity:
To show the effect of acid rain has on buildings, walkways or statues. Be able to discuss what acid rain is, where it comes from and what effect it has.
Acid Rain Demonstration
Acid Rain is created when a large industrial facility burns coal or oil which gives off sulfur, nitrogen and carbon into the air. Their smokestacks carry these waste gases into the clouds where they mix with water vapor to form weak acids – sulfuric, nitric and carbonic acids. When the moisture in the clouds grows heavy enough, it falls as rain - Acid Rain. These toxic clouds are often carried by the prevailing winds for hundreds of miles before they drop their acidic rain or snow. The forests and communities of the northeastern U.S. have been the victim of acid rain that started as air pollution from Midwestern utilities for the last 100 years. Acid rain also corrodes limestone buildings, walkways and statues. Try this Acid Rain Demonstration to see for yourself how acid rain might affect a region.
Objectives:
Students will:
1) Learn the effect acid rain has on buildings, walkways or statues.
2) Be able to discuss what acid rain is.
3) Know where it comes from and what effect it has.
During this investigation, students will be able to:
1. See the chemical reaction of acid on limestone through the vinegar and chalk reaction.
2. Describe what they are seeing and how it relates to acid rain falling on limestone structures.
3. Discuss what causes acid rain and how it gets here.
4. Think about solutions to acid rain.
Supplies:
vinegar, chalk, eye dropper or teaspoon, bowl
Search internet for photos of old statues.
Print any showing acid rain corrosion
Activity:
1. Put chalk piece in the bowl.
2. Using eyedropper or teaspoon, dribble vinegar onto the chalk.
3. The chalk will fizz when vinegar touches it and with enough vinegar added, will then disintegrate.
Discussion: How might acid rain affect:
1) Fish, frogs and salamander eggs in lakes and ponds?
2) Seedling and wildflowers growing in the forest?
When you research information you must cite the reference. Citing for websites is different from citing from books, magazines and periodicals. The style of citing shown here is from the MLA Style Citations (Modern Language Association).
When citing a WEBSITE the general format is as follows.
Author Last Name, First Name(s). "Title: Subtitle of Part of Web Page, if appropriate." Title: Subtitle: Section of Page if appropriate. Sponsoring/Publishing Agency, If Given. Additional significant descriptive information. Date of Electronic Publication or other Date, such as Last Updated. Day Month Year of access < URL >.
Amsel, Sheri. "Acid Rain Demonstration" Exploring Nature Educational Resource ©2005-2024. December 14, 2024
< http://www.exploringnature.org/db/view/Acid-Rain-Demonstration >